All about microbiological analysis.
Microbiological control is a major issue for actors in the food industry who seek to ensure the safety and quality of their products.
As a regulatory reminder: No foodstuff can be put on the market if it is dangerous, harmful to health or unfit for consumption.
To do this, it is important to
- define microbiological criteria adapted to your products
- regularly monitor their compliance.
Let us explain all this to you.
Building microbiological surveillance criteria

The first step in building your microbiological criteria is to know and understand the microbiological risks associated with your products. Indeed, each foodstuff has its own flora and characteristics influencing the growth and survival of microorganisms.
Usually, we gather the criteria of the raw materials and the operations of your processes likely to reduce these initial flora.
For this it is important to refer to the technical data sheets of your suppliers with the guaranteed levels.
This work also allows you to identify whether your customers' requirements, such as specific consumer targets, subsequent process constraints and information on consumption-preservation methods are compatible with this information (germs not taken into account, unsuitable levels, process steps not or insufficiently characterized, etc.).
Once these potential risks have been identified, you can determine the germs to be monitored and the values to be respected.
Check that these values are in accordance with the mandatory criteria defined in the Regulation 2073/2005: you can consider being stricter but not less !
What to do once the criteria are established ?
Regular monitoring of microbiological parameters

Regular monitoring of these parameters will ensure the quality and safety of your products.
How can you do this? By carrying out regular complete microbiological analyses on a large number of samples related to the type of product you want to control the microbiological risks.
The level of contamination assumed or estimated in the construction of the monitoring plan is decisive in determining the frequency of controls.
This can be illustrated as follows
- 50 Salmonella tests per year without positive result allow to define a "true" prevalence between 0 and 7.2%.
- with 1 positive test out of 100 controls: it is between 0 and 5.5%.
- with 10 positive tests out of 1000 controls: it is from 0.4 to 1.9%.
In other words: it is easier to detect a rare phenomenon with a large number of controls, or more commonly in the "no controls: no problems !
How to interpret the results of microbiological analysis?
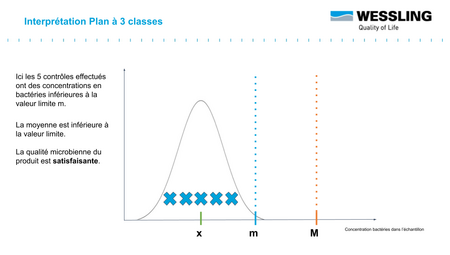
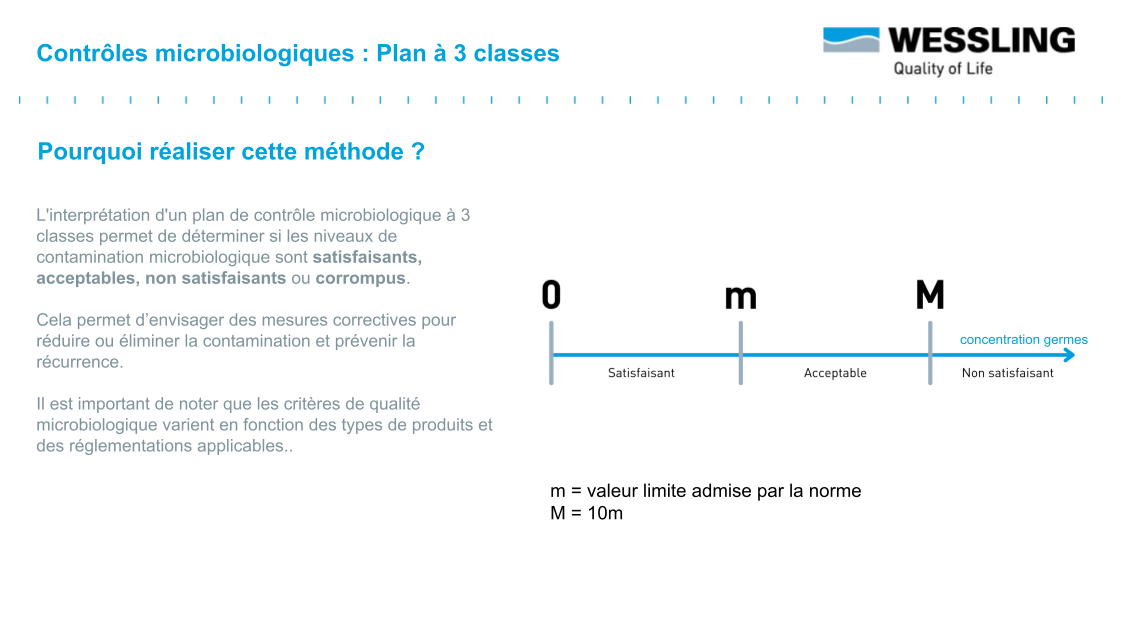
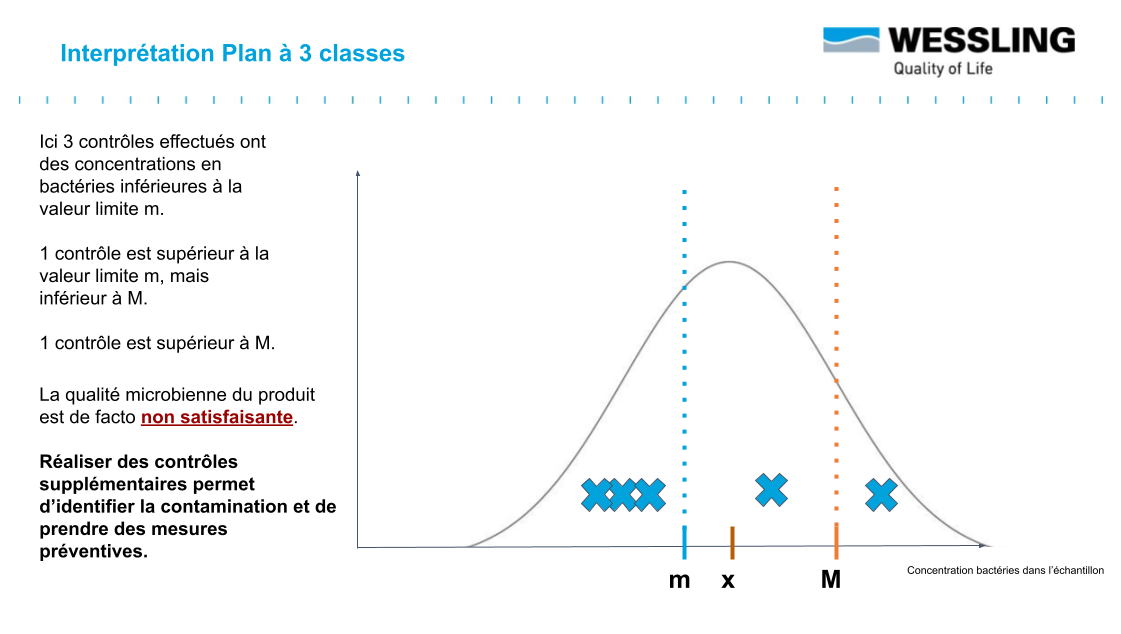
The distribution of germs in a batch is generally considered to follow a Normal distribution which can be represented as in the graphs below.
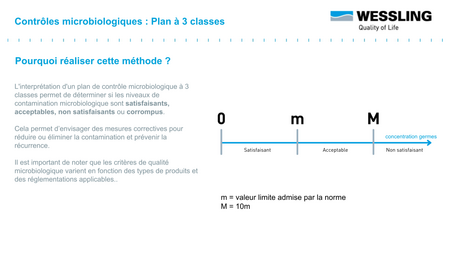
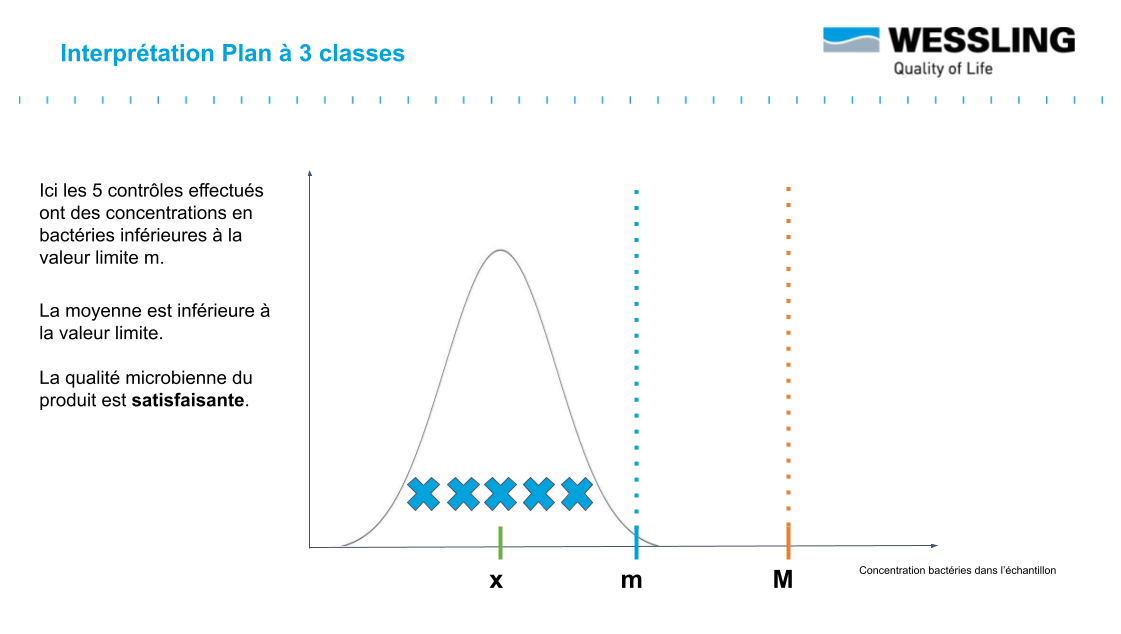
The objective is to have a batch whose mean value ẋ is less than the defined specification m. The value M (usually set at 10 m) represents the tolerance margin that can be applied.
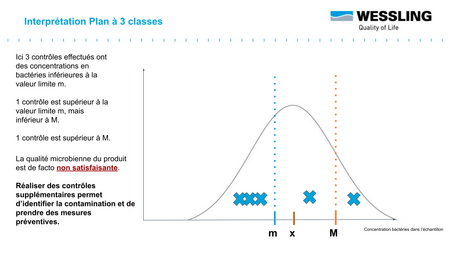
To facilitate interpretation of the results, the three-class design allows results to be classified into three categories:
- compliant : measured result < m
- non-compliant : measured result > M
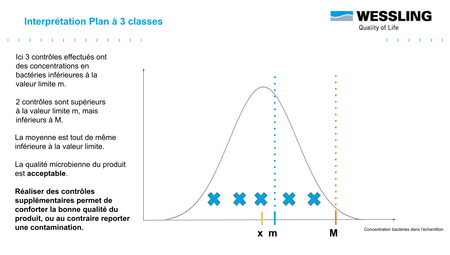
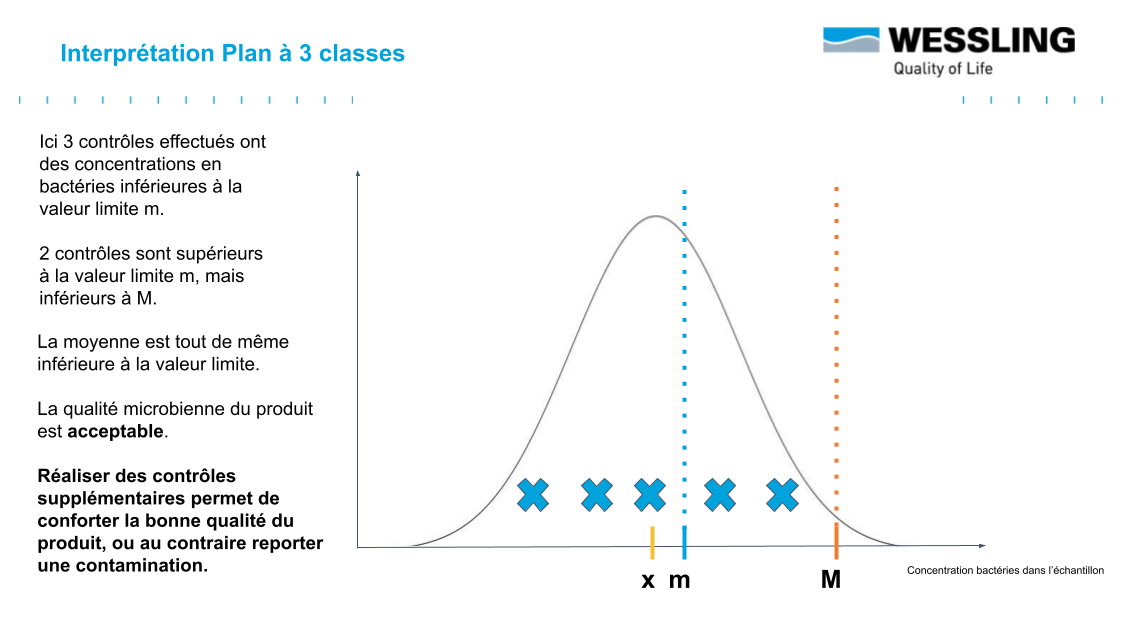
- to be monitored : between m and M ("acceptable result" .... subject to further verification!)
For this last category of measured result, this indicates that you may be in the presence of an "acceptable" result that still falls within the distribution presented above.
Before the acceptable result becomes "accepted" (as is often the case in current practice), it will be necessary to carry out additional analyses to validate the conformity of the batch:
Indeed, statistically, if, for 5 results obtained, at most 2 results are between m and M (and that the 3 others are lower than m), then we can conclude that the average content of the batch is well below m (with a probability of 95%): the batch is thus in conformity !
Because a picture is worth a thousand words, click on the graphs below to better understand the three-class design:
In summary
WESSLING at your service
WESSLING France is at your side to help you build your microbiological criteria and monitor the parameters associated with your product.
We offer you a complete range of microbiological analyses and we also accompany you in the interpretation of the results.
Do not hesitate to contact us to learn more about our microbiological food analysis.
Your dedicated contact
- Léna Soukharevskoff
- +33 6 28 25 30 98
- lena.soukharevskoff@wessling.fr