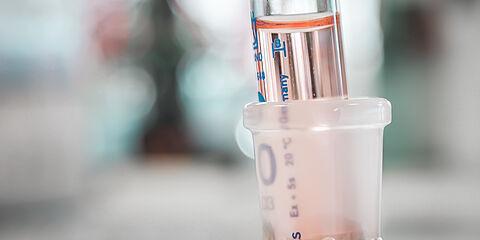Hydroalcoholic products: modification of labelling rules
In the pandemic context due to COVID-19 that is currently affecting Europe and the world, hydroalcoholic products have become a rare commodity. Nevertheless, these skin aseptic solutions with bactericidal, fungicidal and especially virucidal properties are necessary to limit the spread of the COVID-19 virus. To counteract with the current shortage, many cosmetic companies have taken the initiative to produce hydroalcoholic solutions to supply health professionals and all institutions and companies that continue to be active in the daily life of the country.
The composition and effectiveness of hydroalcoholic products

In the context of the Covid-19 pandemic currently affecting Europe and the world, hydroalcoholic products have become a scarce commodity. Many cosmetic companies have taken the initiative to produce hydroalcoholic solutions or gels. The French government has then adapted the regulations in force.
Order of April the 17th 2020: new labelling rules coming into force on 31 May 2020
The order of April the 17th 2020* was updated on April the 21st 2020 to modify the accepted labelling rules for hydroalcoholic products. From May the 31st 2020, the alcoholic degree (%v/v) will have to be displayed on the packaging of hydroalcoholic products. All producers will have to ensure compliance with these new labelling rules.
To comply with these regulatory constraints and ensure the effectiveness of hydroalcoholic products against the SARS-CoV-2 virus, companies producing hydroalcoholic products must therefore quickly have the ethanol concentration in finished products controlled.
At its alcohol laboratory located in Villebon-sur-Yvette (91), France, WESSLING carries out these controls within 24 to 48 hours after receiving the samples and thus provides support to companies producing hydroalcoholic solutions or hydroalcoholic gels.
* Short return on the regulation :
The decree of April the 17th 2020 was modified 3 days later (on April the 21st) when it modified the decree of March the 13th 2020. The latter had been formulated to authorize, by derogation, the temporary placing on the market and use of certain hydro-alcoholic products used as disinfectant biocides for human hygiene.
To continue the activity of the alcohol analysis laboratory at the WESSLING site in Paris, numerous measures have been taken to ensure the health and safety of the company's employees. Workstations in the laboratory have been set up to enable employees to maintain a minimum distance of two meters from each other; each employee is given a new mask, clean gloves and other necessary PPE on a daily basis. In addition, the WESSLING Group has given clear instructions regarding barrier gestures and hygiene rules to be respected. They are repeated daily and the management ensures that they are strictly applied in order to do everything possible to limit the spread of the virus and to ensure the health and safety of the employees.
Your contact
- Nathalie Dozinel
- +33 1 64 47 14 76
- nathalie.dozinel@wessling.fr